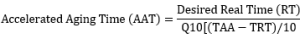QRA has 2 state of the art Labs in Singapore equipped to test your
medical devices from all customers WORLDWIDE.
QRA' s current Clients are from Asia, Australia and United States of
America.
ASTM F1980 - 16
Standard for Accelerated Aging of Sterile Barrier Systems for Medical Devices
ASTM F1980 is a test standard titled, "Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices" is a testing procedure that is used to help with the assessment of the sterile integrity of a package and product designed for medical use.
In order to validate a product and package's Sterile Barrier System (SBS) over the intended storage shelf life, accelerated aging is conducted to evaluate a package and product's long term usability and efficacy. ASTM F1980 is a specific accelerated aging test protocol set forth by ASTM International (American Standards for Test and Measurement International)
It is also advisable to benchmark the product with Shelf Life Test which is to keep the product at the ambient temperature and humidity conditions for the entire duration of the product's useful life.
In summary, 2 different catagories of tests will be carried out simultaneously
1. Accelerated aging test at 55 C 50% for a 3 year shelf life product for 4. 5 months.
2. The 'control' shelf life test to be kept at the full 3 year or 36 months test.
ASTM F1980-21 replaced F1980-16 in December 2021. What changed?
The revisions' change recommends that the use of controlled humidity during acceleratedaging be considered, findings documented, and used during testing if warranted
Purpose of ASTM F1980
To function safely and effectively, medical devices must maintain their sterile integrity throughout their shelf-life. However, over time, the physical properties of the materials may degrade in certain environments and, as a result, may negatively impact the safety and efficacy of the product. Because they endure so many unique environments while being transported,used, and stored,
Medical devices must undergo shelf life testing in addition to sterile integrity testing. Hence the 2 tests above must be run simultaneously.
ASTM F1980 specifically evaluates the aging process of a product along with its package and how it impacts sterility and shelf-life.
Acing tests simulate these conditions by exposing the materials to elevated temperatures for shorter periods of time to represent an equivalent real time shelf life duration. ASTM F1980 testing offers valuable safety and performance insights to manufacturers.
With a greater understanding of the aging process on a product and its package system as the result of testing, manufacturers can and do make more informed decisions regarding the handling, storing, and use of the product. Additionally, precise aging tests achieved by tight temperature tolerances offer enhanced assurance among regulatory agencies and consumers.
QRA Lab 1 at 21 Toh Guan Road East,
04 - 02 Toh Guan Centre
Singapore 608586
12 Top Quality Environmental Chambers with Calibration and Full Reports
1st Class Service with Competitive Pricing Worldwide
Frequently Asked Questions :
What is the procedure for an accelerated aging test for medical devices?
In accelerated aging tests for medical devices, a material or Sterile Barrier System (SBS) is exposed to elevated temperatures for a condensed amount of time. By exposing the testing materials to more extreme conditions during a shorter time frame, researchers can evaluate how a product will age under normal conditions without waiting for the entire desired duration. Armed with this knowledge, manufacturers can determine shelf life, storage, in-use, and transportation parameters more accurately for their product.
Accelerated aging is a standard practice in the medical device industry for determining shelf life parameters by accelerating the effects of time on a Sterile Barrier System (SBS).
The accelerated aging process is based on the relationship between temperature and reaction rate, in which the reaction rate increases as the temperature rises. The Arrhenius Equation is the basic formula used for an accelerated aging test for medical devices is:
Accelerated Aging Time (AAT)= Desired Real Time (RT) divided by the Accelerated Aging
Factor (AAF)
ln summary
Every 10 C increase in ageing temperature shortens the AAT by half.
Therefore if you have a product whose expected shelf life is 3 years or 36 months, the device is expected to 'age' or experience :
> for ambient storage 25 C ; keeping the product in a chamber at 35 C, the product is expected to 'age' in 18 months.
> for ambient storage 25 C ; keeping the product in a chamber at 45 C, the product is expected to 'age' in 9 months.
> for ambient storage 25 C ; keeping the product in a chamber at 55 C, the product is expected to 'age' in 4.5 months.
NOTES
The calculated AAT is typically rounded up to the nearest whole day.
QRA does not recommend aging packaging materials at temperatures exceeding +65°C. Common Accelerated Aging temperatures (TAA) are +50°C, +55°C, and +60°C.
Ambient temperature (TRT) is typically between +20°C to +30°C. A temperature of +25°C is a more conservative approach.
The aging factor is typically between 1.8 - 2.5 with a value of 2.0 being the most common accepted value.
To perform ASTM F1980 accelerated aging tests for medical devices, the laboratory facility must identify the Q10 value of the testing sample. The Q10 temperature coefficient is a measure of how quickly a material system changes when the temperature is increased by +10 C.
QRA has 2 state of the art Labs in Singapore equipped to test your
medical devices from all customers WORLDWIDE.
QRA's current Clients are from Asia, Australia and United States of
America.
QRA Lab 2 at 7 Perahu Road
Singapore 718 836
What are some of the parameters for ASTM F1980 testing?
ASTM International sets forth specific test parameters to ensure consistent testing across different lab facilities. The basic parameters for ASTM F1980 include the following:
1. Accelerated Aging Temperature
2. Humidity (F1980 - 21)
3. The quantity of product testing samples
After the Accelerated Aging Tests, it is advisable to send your medical products to
a) Peel Test
b) Bubble (full immersion) Test
to determine the Confidence and Reliability Levels. QRA can advise you on these matters.
Arrhenius Equation ; What is it ? Why is it Useful ?
Using the Arrhenius Equation, the TRT should accurately reflect the actual product storage and in-use conditions, generally between 20°C and 30°C.
Accelerated aging temperature should be identified prior to testing. This is done by having in-depth knowledge of your materials, product, and packaging. It is not recommended to exceed +65 °C.
The need for controlled humidity during accelerated aging should be identified prior to testing; if materials are subject to moisture degradation, 45% - 55% RH is suggested. This input should be determined with your material providers' assistance.
A Q10 factor needs to be determined, which involves testing materials at various temperatures and defining the differences in reaction rate for a 10° change in temperature. A typical Q10 factor used during testing is 2.
Accelerated aging factor should be specified using the following equation:
AAF = Q10 (TAA-TRT)/10
QRA's sales professionals can walk you through your product ageing test needs.
What is the best temperature to use for an ASTM F1980 test?
The ASTM F1980 standard suggests using an accelerated aging temperature below 60°C. Aging your product at a greater temperature provides the advantage of a faster simulation of the aging interval, but this comes with risks for particular products and packaging materials. Medical devices are often engineered with delicate materials that may drastically change when exposed to temperatures exceeding +60°C. Finding out if your medical product or device may be adversely affected by long periods of high heat or low humidity is a good place to start when choosing the best accelerated aging temperature.
QRA's experts can help you define the ideal temperature parameters for your products and packaging.
Common Accelerated Aging Temperatures: 50 C, 55 C, 57 C or 60 C
Does the F1980-21 version require using controlled humidity during accelerated aging?
In short, humidity is not a required element of accelerated aging. The recent version suggests that humidity conditions in the aging study be defined before starting aging studies. If RH will not be controlled, the rationale for exclusion should be documented.
What is the best humidity level to use for accelerated aging?
Humidity usage is dependent on the materials used in your product and packaging, how moisture impacts them, and other environmental factors. If humidity during accelerated aging is to be controlled,
How do you evaluate the ASTM F1980 test, post-aging?
After a testing sample has undergone the accelerated aging process, its physical properties and package integrity will be compared against various aging time points.
This includes as per ASTM F1886,
1. Peel Testing
2. Bubble Test
3. Dye Test
My products must comply with the new F1980-21 revision. What do you recommend?
Whether you have a released medical device or a new product in development, QRA sales experts can help. Our recommendations will be based on your company's unique product, expected shelf life and how much you value your brand.
Talk to The Experts. Talk to QRA
Consultancy is at NO COST.
Quotations are Free and Prompt. Prices are Inxpensive.
Service is World Class.
Please contact : Email : mark@qra.com.sg
Email : qrasales@qra.com.sg
Website : www.qra.com.sg