What is the meaning of 'shelf life' ?
Shelf-life testing demonstrates the ability of a packaging system to maintain sterile integrity and strength over a defined period of time, and is indicated by labelling the product with a use by or expiry date.
Shelf life is defined as the length of time that a product may be stored without becoming:
- Unfit for use
- Unfit for consumption
- Unfit for sale
What is accelerated aging?
Accelerated aging is an artificial procedure used for establishing the shelf life to estimate the useful lifespan of a product when actual lifespan data is unavailable in an expedited manner.
Accelerated aging is conducted by simulated exposure of the product to elevated temperatures and realistic relative humidity levels for the equivalent period claimed for product expiration. The provisional accelerated aging expiration date will remain until real time aging results are acquired.
Typical Accelerated Aging Temp and Humidity Conditions
1. 55 C 50% RH
2. 60 C 50 % RH
Typical Duration of Tests
1. 18 months
2. 9 months
3. 4.5 months
What is Real Time aging?
Real time aging is a stability testing process used to provide real data to determine the shelf life and the effects of aging on materials over time. The packaged product is exposed to storage conditions such as temperature and relative humidity to determine its shelf life. These studies are typically carried out at ambient temperature between 25°C to 30°C.
Humidity is also controlled with settings of between 40 to 60% being the most common settings.
How is accelerated aging calculated according to ASTM F1980 ?
Accelerated aging techniques are defined on the basis that the chemical reactions involved in the deterioration of materials follow the Arrhenius reaction rate. This states that a 10°C temperature increase results in approximately two times an increase in the rate of the aging process. The accelerated aging process is based on the relationship of temperature and reaction rate where an increase in temperature increases the reaction rate.
The level of relative humidity (RH), should be included at realistic levels. When controlling humidity during an accelerated aging study, it is suggested in the standard to use a realistic level between 45% RH and 55% RH unless there are documented reasons for using other humidity levels. If other levels are used, a rationale should be documented.
- It is not recommended by the standard to age packaged products at temperatures above 60°C unless knowledge of the materials shows otherwise
- The calculated duration is normally rounded up to the nearest whole day
- Ambient temperature Real Time Temperature (TRT) is typically between 25°C to 30°C
- The aging factor of 2.0 is the most common value but other levels between 1.8 – 2.5 may be used once sufficient knowledge of the materials are known and can be justified
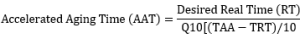
Product temperature storage labelling considerations
Where a product is labeled with specific storage temperature range, the upper temperature listed should be used when calculating the accelerated aging duration. This is instead of the normal ambient temperature (TRT), which is typically 25°C or 30°C. If the labelled range is 15°C to 35°C, then the 35°C should be used as the ambient temperature TRT in the calculation.
Timepoints:
It is recommended that minimum of two shelf-life timepoints are used. This provides a backup if post aging tests do not meet acceptance criteria for a particular timepoint.
QRA offers a range of accelerated aging chambers, which can accommodate both large pallets and single shipping units.
Stability Testing
What are stability studies?
Stability studies are conducted to evaluate the quality of a pharmaceutical or drug product over time to evaluate the influence of various environmental factors such as temperature, humidity, and light. Typically, under accelerated conditions, this simulates the effects of long-term storage in a shorter period of time.
STERIS also offers stability testing capabilities in accordance with ICH guidelines for pharmaceutical manufacturers.
Conditions include:
- 5°C
- 30°C, 60% RH
- 30°C, 65% RH
- 30°C, 75% RH
- 40°C, 75% RH




No comments:
Post a Comment